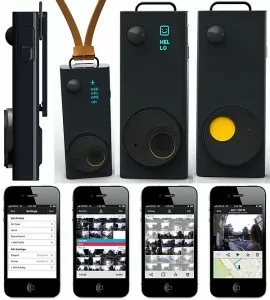
Pennsylvania-based Strados Labs has received the FDA’s 510(k) clearance for its Strados RESP, a platform used to record, measure and assess lung sounds remotely.
Read more Kaia Health Partners With Chiesi Group to Commercialize Kaia COPD Rehabilitation App in Europe
The RESP™ system has been used in clinical trials to help researchers quantify changes in lung sounds over time to compare against treatments, patient-reported outcomes, and other vital signs. The RESP™ can capture, store, and make available for clinical analysis these key changes in a patient’s lung sounds remotely with a small body-worn sensor and a cloud-based, HIPAA compliant software system, says a press release.
Dr. Mitchell Glass, the Chief Medical Officer of Strados Labs, said, “This wearable technology will allow us to follow our patients in healthcare settings more effectively, by providing regular interval listening between clinician visits, by archiving the patient’s lung sounds for future comparisons and by reducing the variability in auscultatory documentation that presents a major problem both in patient care and in clinical trials.”
The company is currently working with research organizations and pharmaceutical companies to capture lung sound measurements as primary and exploratory endpoints to support their trials. Strados Labs sees the use of RESP™ in trials as a way to learn more about patient-reporting outcomes (ePRO) to help sponsors better understand how treatments and therapies impact patient symptoms like coughing or wheezing objectively. This system for remote lung measurement aligns with the sponsor goals of minimizing patient burden, maintaining patient privacy, and decentralizing trial data collection to optimize recruitment and retention.
 Tele-intensivists can listen to a patient’s lung sounds even when a bedside nurse is not available. Clinicians can also live stream for a tele-consultation or in a rural telehealth program. (Strados Labs)
Tele-intensivists can listen to a patient’s lung sounds even when a bedside nurse is not available. Clinicians can also live stream for a tele-consultation or in a rural telehealth program. (Strados Labs)
“We are excited to finally bring the RESP™ technology to clinicians, scientists and patients who need it most,” said Co-founder & CEO Nick Delmonico. “The FDA clearance is a major milestone for our early-stage company. Our team worked tirelessly and overcame obstacles, many that were out of our control due to COVID19, to achieve this goal during a particularly challenging year for small businesses. We are thrilled to be one step closer to our mission of making every breath count.”
The RESP technology can be integrated into various health data systems, including telehealth, eICUs, clinical trial management platforms, and telemetry across healthcare settings. This is the company’s first FDA clearance.